
Water in Compressed Air Calculations
To continue on the topic of ‘water in your compressed air’ (the other two articles are here and here), let’s examine how we can calculate the amount of water that is generated in a typical compressed air system. I promise, you will be surprised! ## What influences the amount of water in a compressed air system
 There are a few things that influence the amount of water in your compressed air system. The main factors are the intake relative humidity and temperature, the end pressure and the end temperature. The intake relative humidity and temperature gives us the total amount of water vapor that enters the compressed air system. In other words, we can calculate that absolute humidity of the incoming air in grams per cubic meter. The output pressure and temperature gives us the maximum amount of water vapor that the compressed air can hold, again in grams per cubic meter. If the incoming water vapor is more than what the compressed air can hold, some of it will condense into liquid water, once compressed. If the holding capacity of the compressed air is big enough to hold all the incoming water vapor, then no water vapor will condense into liquid water: your compressed air stays dry. ## Static example
There are a few things that influence the amount of water in your compressed air system. The main factors are the intake relative humidity and temperature, the end pressure and the end temperature. The intake relative humidity and temperature gives us the total amount of water vapor that enters the compressed air system. In other words, we can calculate that absolute humidity of the incoming air in grams per cubic meter. The output pressure and temperature gives us the maximum amount of water vapor that the compressed air can hold, again in grams per cubic meter. If the incoming water vapor is more than what the compressed air can hold, some of it will condense into liquid water, once compressed. If the holding capacity of the compressed air is big enough to hold all the incoming water vapor, then no water vapor will condense into liquid water: your compressed air stays dry. ## Static example
Let’s first take a look at a static example. By this I mean we will compressed a fixed amount of air, as if we were in a laboratory. Let’s say we have a 7 bar compressed air system. Since we normally talk relative pressures in compressed air land, that’s 7 bar relative pressure or 8 bar absolute pressure. This means we need to compress the ambient air 8 times (from 8 m³ to 1 m³ for example). The pressure will increase from 1 bar absolute to 8 bar absolute. Air Compression:- Volume decreases 8 times. From 8 m³ to 1 m³
- Pressure increases 8 times. From 1 bar to 8 bar (absolute)
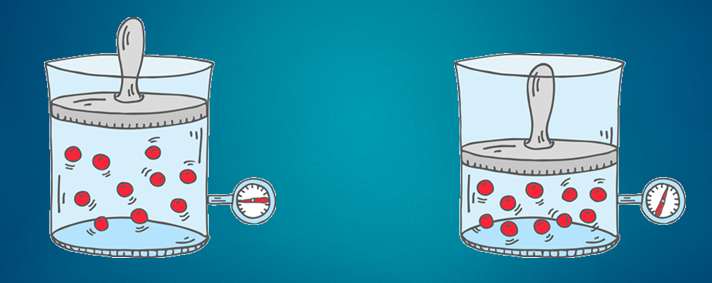 But we don’t just compress air. We compress air that holds some water vapor. The total (absolute) amount of water vapor of the incoming air depends on the temperature and the relative humidity of that air. As we compress the air, the water vapor that was present in the 8 m³ is now squeezed into 1 m³. (we assume the temperature constant. In reality it isn't, but if we give the compressed air time to cool down, it will again be the same temperature as it was before compression). Now let’s see what happens with the water vapor. Let’s say we had air of 20°C and a relative humidity of 50%. Air of 20°C can hold 17 g/m³ (grams per cubic meter). Since our air is 50% relative humidity, it holds half of that: 8.5 g/m³.
But we don’t just compress air. We compress air that holds some water vapor. The total (absolute) amount of water vapor of the incoming air depends on the temperature and the relative humidity of that air. As we compress the air, the water vapor that was present in the 8 m³ is now squeezed into 1 m³. (we assume the temperature constant. In reality it isn't, but if we give the compressed air time to cool down, it will again be the same temperature as it was before compression). Now let’s see what happens with the water vapor. Let’s say we had air of 20°C and a relative humidity of 50%. Air of 20°C can hold 17 g/m³ (grams per cubic meter). Since our air is 50% relative humidity, it holds half of that: 8.5 g/m³. 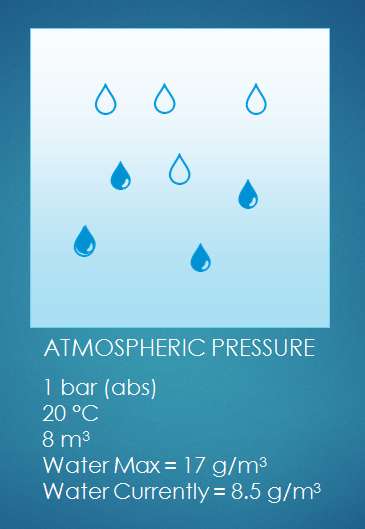 Now let’s do some calculations... Compressed air created We compress the air to 7 bar relative pressure. That’s 8 bar absolute pressure. In the process we reduced the volume 8 times. Our 1 m³ intake air becomes 1/8 m³ compressed air, or 0.125 m³ compressed air. Water content of the compressed air Each cubic meter of inlet air contains 8.5 grams of water vapor. We compress the air: we push 8 cubic meters into 1 cubic meters of compressed air. This means our compressed air has a water content of 8.5 * 8 = 68 g/m³ (grams of water per cubic meter of air). Maximum water vapor content in compressed air The hold capacity of air depends on it’s temperature. This means that a cubic meter of ambient air of 20°C can hold the same amount of water vapor as a cubic meter of compressed air of 20 °C. The pressure only has a tiny bit of influence on the water vapor holding capacity of the air. We can forget about it here. We already saw that 1 cubic meter of 20°C can hold a maximum of 17 grams of water vapor. Our compressed air of 20°C can hold the same 17 grams of water vapor maximum. Water vapor vs liquid water in our compressed air As we see, the water content of the compressed air is bigger than the maximum water vapor holding capacity of the air! We could say the relative humidity is 400% (impossible!) - Max = 17 g/m³
Now let’s do some calculations... Compressed air created We compress the air to 7 bar relative pressure. That’s 8 bar absolute pressure. In the process we reduced the volume 8 times. Our 1 m³ intake air becomes 1/8 m³ compressed air, or 0.125 m³ compressed air. Water content of the compressed air Each cubic meter of inlet air contains 8.5 grams of water vapor. We compress the air: we push 8 cubic meters into 1 cubic meters of compressed air. This means our compressed air has a water content of 8.5 * 8 = 68 g/m³ (grams of water per cubic meter of air). Maximum water vapor content in compressed air The hold capacity of air depends on it’s temperature. This means that a cubic meter of ambient air of 20°C can hold the same amount of water vapor as a cubic meter of compressed air of 20 °C. The pressure only has a tiny bit of influence on the water vapor holding capacity of the air. We can forget about it here. We already saw that 1 cubic meter of 20°C can hold a maximum of 17 grams of water vapor. Our compressed air of 20°C can hold the same 17 grams of water vapor maximum. Water vapor vs liquid water in our compressed air As we see, the water content of the compressed air is bigger than the maximum water vapor holding capacity of the air! We could say the relative humidity is 400% (impossible!) - Max = 17 g/m³
- Actual = 68 g/m³
The difference will condense into liquid water, it ‘rains’ in our compressed air system. The result is that we have 17 gr of water vapor (the maximum, 100% relative humidity, or saturated air), and we have 68 – 17 = 51 grams of liquid water in our compressed air. 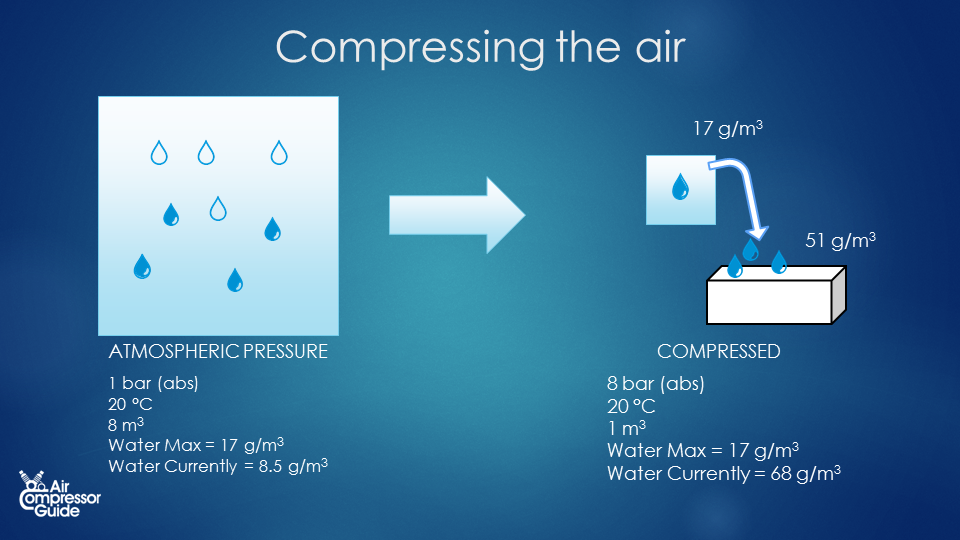 All in one table: Before compression After compression Pressure [bar, absolute] 1 8 Volume 8 m³ 1 m³ Temperature [°C] 20 °C 20 °C (after cooling down) Max. humidity [absolute] 17 g/m³ 17 g/m³ Actual humidity [absolute] 8.5 g/m³ 68 g/m³ Actual Humidity [relative] 50% 400% Total water present 68 g 68 g Water as vapor 68 g 17 g Liquid water 0 g 51 g So for every 8 cubic meters of ambient air that we compressor, we generate 1 cubic meter of compressed air at 7 bar (relative pressure, 8 bar absolute pressure), with a water vapor content of 17 g/m³ . And we generate 51 g of liquid water. That’s 0.051 liters. ## Compressed Air System Example
All in one table: Before compression After compression Pressure [bar, absolute] 1 8 Volume 8 m³ 1 m³ Temperature [°C] 20 °C 20 °C (after cooling down) Max. humidity [absolute] 17 g/m³ 17 g/m³ Actual humidity [absolute] 8.5 g/m³ 68 g/m³ Actual Humidity [relative] 50% 400% Total water present 68 g 68 g Water as vapor 68 g 17 g Liquid water 0 g 51 g So for every 8 cubic meters of ambient air that we compressor, we generate 1 cubic meter of compressed air at 7 bar (relative pressure, 8 bar absolute pressure), with a water vapor content of 17 g/m³ . And we generate 51 g of liquid water. That’s 0.051 liters. ## Compressed Air System Example
Now let’s do the same for a real compressed air system. In a compressed air system, we don’t compressed a certain volume of air once, we compress air continuously. The capacity of air compressors is expressed as “Free Air Delivery” or FAD. FAD is basically the amount of ambient air a compressor takes in, calculated in standard conditions. From static example to real compressed air system Let’s keep the same ambient air conditions as before: 20 °C and 50% Relative Humidity. We change from our static example of compressing 1 cubic meter once, to compressing many cubic meters every minute. The resulting amount of water that’s generated isn’t expressed in ‘liters’, it’s expressed as “liters per minutes”. Real example Let’s calculate the amount of water that is generated in a compressed air system every day. To make it easy to wrap your head around it, we can just imagine that we have a huge compressed air receiver and we store all the compressed air for the day inside that compressed air receiver. Remember our static example: Before compression After compression Pressure [bar, absolute] 1 8 Volume 8 m³ 1 m³ Total water present 68 g (8.5 g/m³) 68 g Water as vapor 68 g 17 g Liquid water 0 g 51 g Let’s change that from a static once-off compression to a real compressed air system, where a compressor pumps in a certain amount of air every minute. Let’s take an Ingersoll Rand IRN45K rotary screw air compressor. This compressor has a capacity of 7 m³ per minute. That’s intake air (FAD). We can say that it takes in 7 m³ per minute. Air intake per day: Our compressor takes in 7 m³/min. That’s 7 * 60 *24 = 10,080 m³ per day (of ambient air). Water present in intake air: The air in our example contains 8.5 g/m³ (g per cubic meter of air) of water vapor. 10,080 * 8.5 = 85,680 grams of water vapor per day, that this compressor takes in with the ambient air. Compressed air created We compress the air to 7 bar relative pressure. That’s 8 bar absolute pressure. In the process we reduced the volume 8 times. Our 10,080 m³ intake air becomes 1,260 m³ compressed air (per day). So if we imagine we store the all the compressed air for the day in a big air receiver, that air receiver would need to be 1,260 m³ (that's huge! An average compressed air receiver is 1 to 3 m³, but of course normally we use the compressed air we created directly. Total water content of compressed air We created 1,260 m³ of compressed air, and that compressed air contained 68 g/m³ (see our static example). In total we now how 1,260 * 68 = 85,680 grams of water in the compressed air that we generated during that day. Another way to look at this is that the total water content (vapor and liquid) before and after compression stays the same. The water doesn’t just disappear somewhere. We already saw that we took in 85,680 grams of water per day (see calculation ”Water present in intake air”). That’s correct! Total water content before and after compression is that same. Water vapor vs liquid water in our compressed air We saw in the static example that the compressed air can hold 17 grams of water per cubic meter of compressed air. In total, we created 1,260 m³ of compressed air in a day. The amount of water vapor our compressed air can hold is 1,260 * 17 = 21,420 grams of water in total. The problem is: we have a total water content of 85,680 grams. Just like in our static example, the difference condenses into liquid water. 85,680 – 21,420 = 64,260 grams of water per day. That’s 64.2 liters! Total water content (liquid and vapor) = 85,680 grams Water vapor = 21,420 grams (the maximum, 100% relative humidity) Liquid water = 64,260 grams That is 64 liters of liquid water per day in our compressed air system!## Summary
Let’s put all that in 1 table: Before compression After compression Pressure [bar, absolute] 1 8 Volume 1 m³ 1/8 m³ ( 0.125 m³) Temperature 20 °C 20 °C Per m³ Water present 8.5 g/m³ 68 g / m³ Water as vapor 8.5 g/m³ 17 g / m³ Water as liquid 0 g/m³ 51 g / m³ Per day Air volume 10,080 m³ 1,260 m³ Water present 85,680 g 85,680 g Water as vapor 85,680 g 21,420 g Water as liquid 0 g 64,260 g = 64 liter
The problem.
We saw that 64.2 liters of water are generated in our example compressed air system every single day. We need to remove the water from our compressed air to prevent damage to our compressed air users. We do that by simple condensate traps and condensate drains. But what’s the problem here? There are two problems: 1) Our compressed air is now 100% relative humidity. It is ‘saturated air’. It’s just on the edge of condensing into liquid, or raining down. 2) The air leaving the compressor isn’t cool, it's still hot. In our example, we assumed that the compressed air cools down back to ambient temperature. 20°C in our example. But in reality, the air that leaves the compressor is much warmer, probably around 40 °C If will take some time to cool back down completely to 20 °C. Maybe that happens at the end of your compressed air piping, near your air consumers. This means that not all water will condense to liquid water in or near your air compressor: a lot of water will cool down further down the line. That means: water in your compressed air! Updated example Let’s update our example compressed air system. A typical rotary screw air compressor runs at a temperature of 80 °C. Before the air leaves the air compressor, it is cooled down in the after cooler. That’s where a lot of condensation takes place. So you will always see a condensate trap with a condensate drain right downstream of the after cooler. [caption id="attachment_12791" align="aligncenter" width="460"]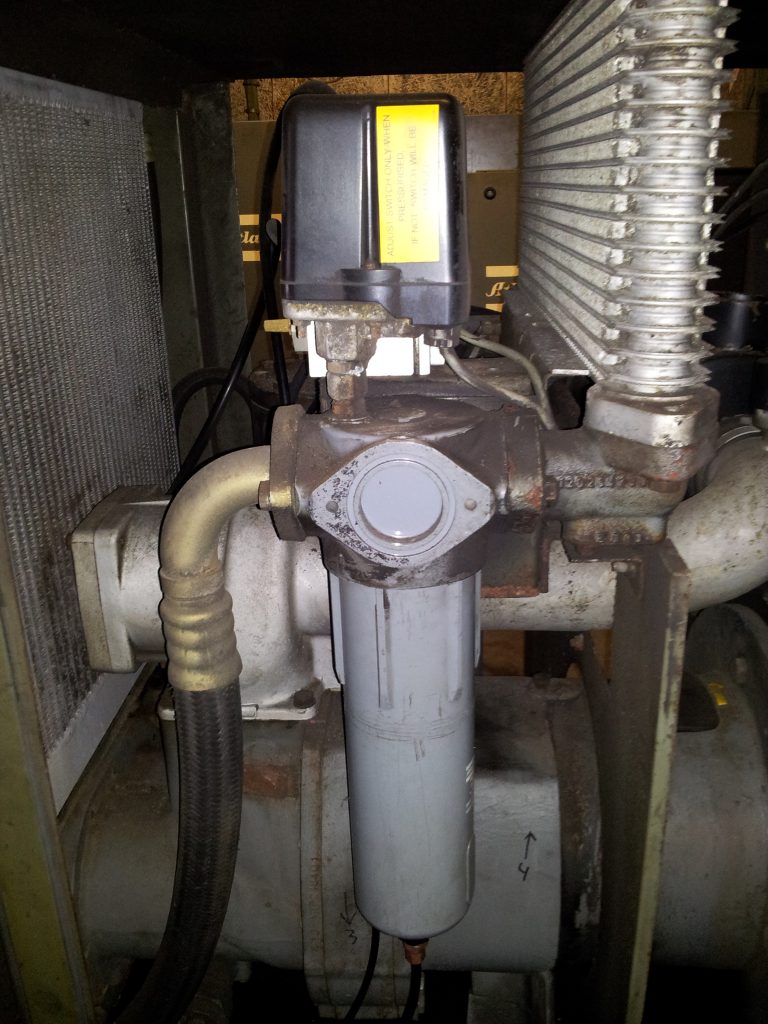 Condensate trap (middle) installed right downstream of the after-cooler (right)[/caption] Let’s say the air is cooled down to 40 °C. And it cools down to 20 °C only later, somewhere in our compressed air system. How much liquid water is removed at the air compressor, and how much liquid water will be present in our compressed air piping system? Compressed Air at 40 °C. As you remember, the water vapor holding capacity of air depends on it’s temperature. Air of 40 °C can hold 51 grams per cubic meter of air. That’s much more water vapor compared to air of 20 °C (17 g/m³). After cooler condensation How much water vapor condenses to liquid water in our after cooler? Remember, we created 1,260 m³ of compressed air. Together it can hold 1,260 * 51 = 64,260 grams of water vapor (at 40 °C) But, we push in 85,680 grams of water in a day. The difference condenses to liquid water: 85,680 – 64,260 = 21,420 grams, or 21.4 liters. Remember, the compressed air that leaves our compressor, after the after cooler, is 40 °C and 100% Relative Humidity. As this air cools down, more and more water vapor will condense into liquid water. How much? Lets find out. Piping system condensation. At the end of our compressed air piping system, the air has cooled back down to 20°C. We already saw that our compressed air of 20 °C (1,260 m³ total for the day) can hold 1,260 * 17 = 21,420 grams of water vapor. The difference between the 40 °C compressed air and the 20 °C will condense to liquid water in our compressed air system. For our total of 1,260 m³ compressed air for the day, that’s: 64,260 – 21,420 = 42,940 grams = 42.9 liters per day So in our more realistic compressed air system, these are the stats per day: Compressed air created = 1,260 m³ Total water content generated = 85,680 grams of water Condensation in after cooler = 21,420 grams, or 21 liters. Condensation in piping system = 42,940 g, or 43 liters. Remember, this is per day!
Condensate trap (middle) installed right downstream of the after-cooler (right)[/caption] Let’s say the air is cooled down to 40 °C. And it cools down to 20 °C only later, somewhere in our compressed air system. How much liquid water is removed at the air compressor, and how much liquid water will be present in our compressed air piping system? Compressed Air at 40 °C. As you remember, the water vapor holding capacity of air depends on it’s temperature. Air of 40 °C can hold 51 grams per cubic meter of air. That’s much more water vapor compared to air of 20 °C (17 g/m³). After cooler condensation How much water vapor condenses to liquid water in our after cooler? Remember, we created 1,260 m³ of compressed air. Together it can hold 1,260 * 51 = 64,260 grams of water vapor (at 40 °C) But, we push in 85,680 grams of water in a day. The difference condenses to liquid water: 85,680 – 64,260 = 21,420 grams, or 21.4 liters. Remember, the compressed air that leaves our compressor, after the after cooler, is 40 °C and 100% Relative Humidity. As this air cools down, more and more water vapor will condense into liquid water. How much? Lets find out. Piping system condensation. At the end of our compressed air piping system, the air has cooled back down to 20°C. We already saw that our compressed air of 20 °C (1,260 m³ total for the day) can hold 1,260 * 17 = 21,420 grams of water vapor. The difference between the 40 °C compressed air and the 20 °C will condense to liquid water in our compressed air system. For our total of 1,260 m³ compressed air for the day, that’s: 64,260 – 21,420 = 42,940 grams = 42.9 liters per day So in our more realistic compressed air system, these are the stats per day: Compressed air created = 1,260 m³ Total water content generated = 85,680 grams of water Condensation in after cooler = 21,420 grams, or 21 liters. Condensation in piping system = 42,940 g, or 43 liters. Remember, this is per day! 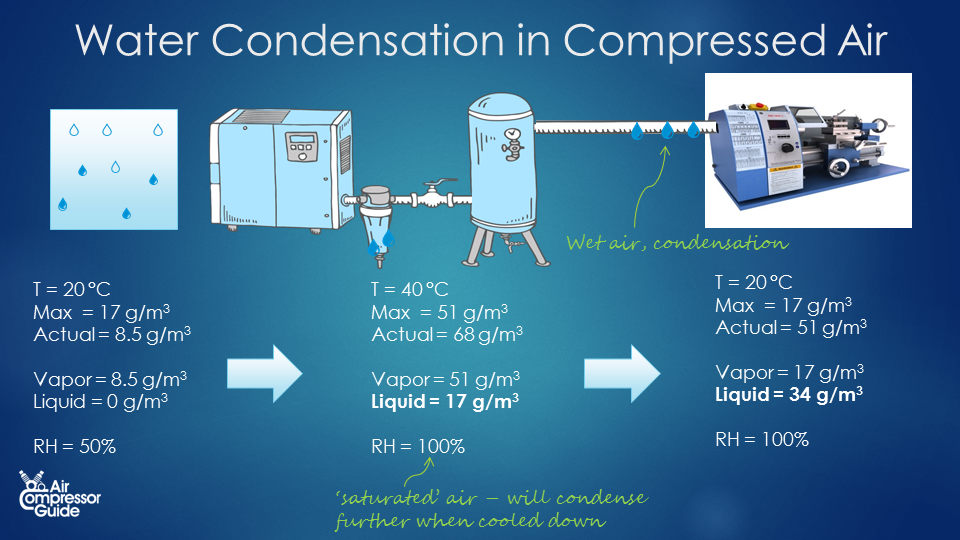 In this example, we see clearly that condensate traps only remove liquid water from the compressed air. They don’t remove any water vapor. We removed all liquid water right after the after cooler in the compressor. The compressed air seemed ‘dry’. But it was 100% relative humidity, or saturated, soaking wet compressed air! After it cooled down more in our piping system, another 42.9 liters of condensate was created! That’s the reason we have air dryers! Air dryers remove water vapor from the air. It actually dries the air, where condensate traps only remove liquid water from the air. ## Update example with air dryer.
In this example, we see clearly that condensate traps only remove liquid water from the compressed air. They don’t remove any water vapor. We removed all liquid water right after the after cooler in the compressor. The compressed air seemed ‘dry’. But it was 100% relative humidity, or saturated, soaking wet compressed air! After it cooled down more in our piping system, another 42.9 liters of condensate was created! That’s the reason we have air dryers! Air dryers remove water vapor from the air. It actually dries the air, where condensate traps only remove liquid water from the air. ## Update example with air dryer.
For fun, let’s add an air dryer to our example system 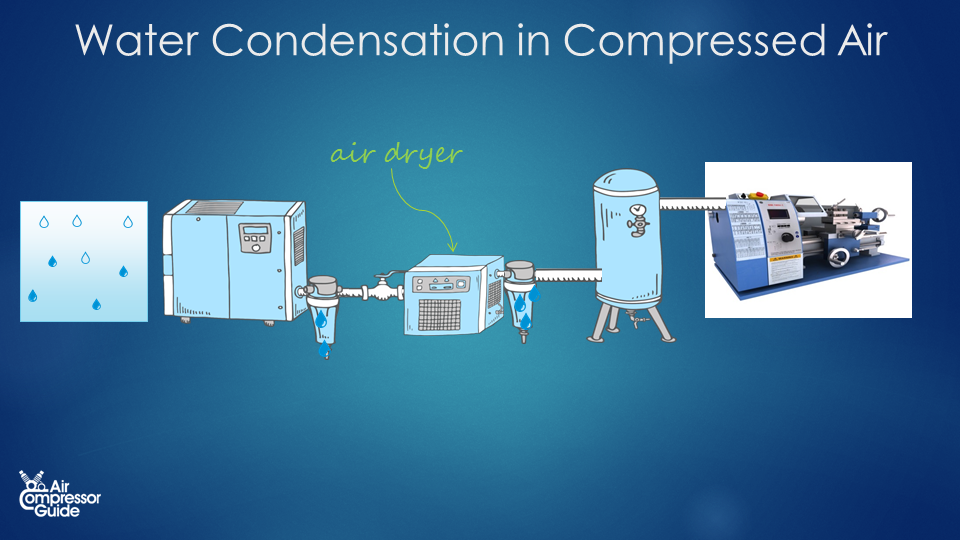 The simplest type of compressed air dryer is a so-called refrigerated compressed air dryer. What they do is, they cool down the air to around 4 °C and then re-heat the air back close to the original temperature before the air dryer. As the air cools down, more and ore condensate forms. The condensate is removed using a simple condensate trap and drain before the air is re-heated again. Condensation in air dryer The typical dewpoint temperature of a refrigerated compressed air dryer is 4 °C. At 4°C, air can hold only 6.25 g/m³ (grams of water vapor per cubic meter of air). Our compressed air leaving the air compressor at 40 °C contained 51 grams of water vapor per cubic meter of compressed air. This means 51 – 6.25 = 44.75 grams of water vapor turns into liquid water, while 6.25 g remain as water vapor (that is, the maximum at 4 °C). In our example compressed air system, we created 1,260 m³ of compressed air per day, thus we have 1,260 * 44.75 g = 56,385 grams of condensate water per day. That’s a little more than 56 liters per day! We are removing water from the compressed air! We can already see that we are actually removing more water from the air with our air dryer. Without air dryer, we had 21 liters condensate water in our after cooler, and 42.9 liters in our piping system. Total is 21 + 42.9 = 63.9 liters. Now with out air dryer, we still have the 21 liters of condensate water in our after cooler, but we also removed 56 liters in our compressed air dryer! That’s a total of 21 + 56 = 77 liters of water removed from the compressed air! Humidity of our compressed air after the air dryer Now just for fun, let’s calculate the relative humidity after the air dryer. Let’s say that the outlet temperature of the air dryer is 30 °C. That’s somewhere in between the inlet temperature of 40°C and the ambient temperature of 20°C. At 30°C, air can contain a maximum of 30 g/m³ (grams of water vapor per cubic meter of air). Most water condensed into liquid water when we cooled it down to 4 °C. Only 6.25 g/m³ remained in the compressed air. So right after the air dryer, we have air of 30 °C with only 6.25 g/m³ of water vapor. While air of 30 °C can hold a maximum of 30 g/m³. We can already see we have created dry compressed air! In fact, our air has a Relative Humidity of only 21%. And we can also say, no more condensate water will form as long as the compressed air stays above 4 °C.
The simplest type of compressed air dryer is a so-called refrigerated compressed air dryer. What they do is, they cool down the air to around 4 °C and then re-heat the air back close to the original temperature before the air dryer. As the air cools down, more and ore condensate forms. The condensate is removed using a simple condensate trap and drain before the air is re-heated again. Condensation in air dryer The typical dewpoint temperature of a refrigerated compressed air dryer is 4 °C. At 4°C, air can hold only 6.25 g/m³ (grams of water vapor per cubic meter of air). Our compressed air leaving the air compressor at 40 °C contained 51 grams of water vapor per cubic meter of compressed air. This means 51 – 6.25 = 44.75 grams of water vapor turns into liquid water, while 6.25 g remain as water vapor (that is, the maximum at 4 °C). In our example compressed air system, we created 1,260 m³ of compressed air per day, thus we have 1,260 * 44.75 g = 56,385 grams of condensate water per day. That’s a little more than 56 liters per day! We are removing water from the compressed air! We can already see that we are actually removing more water from the air with our air dryer. Without air dryer, we had 21 liters condensate water in our after cooler, and 42.9 liters in our piping system. Total is 21 + 42.9 = 63.9 liters. Now with out air dryer, we still have the 21 liters of condensate water in our after cooler, but we also removed 56 liters in our compressed air dryer! That’s a total of 21 + 56 = 77 liters of water removed from the compressed air! Humidity of our compressed air after the air dryer Now just for fun, let’s calculate the relative humidity after the air dryer. Let’s say that the outlet temperature of the air dryer is 30 °C. That’s somewhere in between the inlet temperature of 40°C and the ambient temperature of 20°C. At 30°C, air can contain a maximum of 30 g/m³ (grams of water vapor per cubic meter of air). Most water condensed into liquid water when we cooled it down to 4 °C. Only 6.25 g/m³ remained in the compressed air. So right after the air dryer, we have air of 30 °C with only 6.25 g/m³ of water vapor. While air of 30 °C can hold a maximum of 30 g/m³. We can already see we have created dry compressed air! In fact, our air has a Relative Humidity of only 21%. And we can also say, no more condensate water will form as long as the compressed air stays above 4 °C. 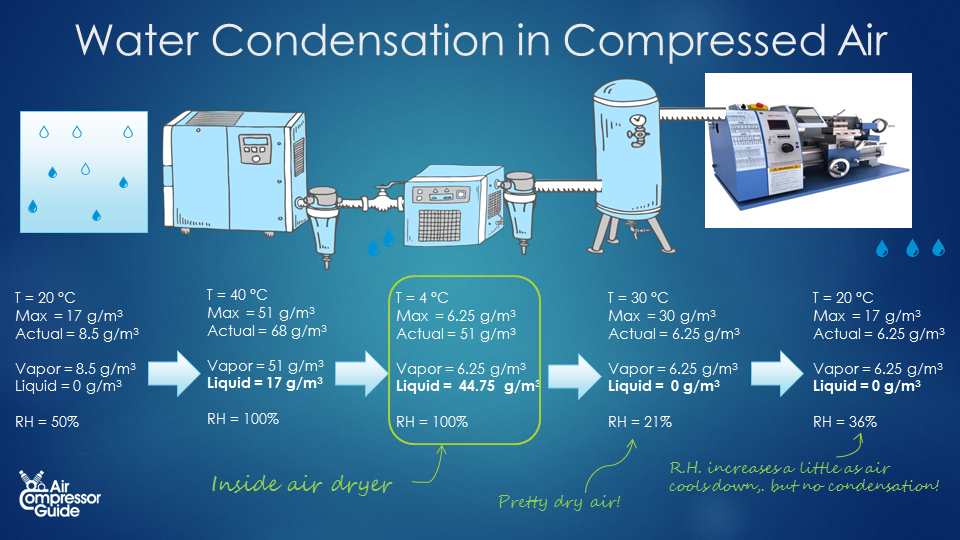 In summary, this is what happens in a day: Before Compression After compression After after cooler Inside dryer After dryer End of compressed air system Pressure [bar, absolute] 1 bar 8 bar 8 bar 8 bar 8 bar 8 bar Volume 10,080 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ Temperature 20 °C 80 °C 40 °C 4 °C 30 °C 20 °C Per m³ Max. water content 17 g/m³ Very high 51 g/m³ 6.25 g/m³ 30 g/m³ 17 g/m³ Water present 8.5 g/m³ 68 g/m³ 68 g/m³ 51 g/m³ 6.25 g/m³ 6.25 g/m³ Water as vapor 8.5 g/m³ 68 g/m³ 51 g/m³ 6.25 g/m³ 6.25 g/m³ 6.25 g/m³ Water as liquid 0 g/m³ 0 g/m³ 17 g/m³ 44.6 g/m³ 0 g/m³ 0 g/m³ Relative Humidity 50% Low 100% 100% 21% 36 % Per day Air volume 10,080 m³ 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ Water present 85,680 g 85,680 g 85,680 g 64,260 g 8,064 g 8,064 g Water as vapor 85,680 g 85,680 g 64,260 g 8,064 g 8,064 g 8,064 g Water as liquid 0 g 0 g 21,420 g = 21 liter 56,196 g = 56 liter 0 0 ## Conclusion
In summary, this is what happens in a day: Before Compression After compression After after cooler Inside dryer After dryer End of compressed air system Pressure [bar, absolute] 1 bar 8 bar 8 bar 8 bar 8 bar 8 bar Volume 10,080 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ Temperature 20 °C 80 °C 40 °C 4 °C 30 °C 20 °C Per m³ Max. water content 17 g/m³ Very high 51 g/m³ 6.25 g/m³ 30 g/m³ 17 g/m³ Water present 8.5 g/m³ 68 g/m³ 68 g/m³ 51 g/m³ 6.25 g/m³ 6.25 g/m³ Water as vapor 8.5 g/m³ 68 g/m³ 51 g/m³ 6.25 g/m³ 6.25 g/m³ 6.25 g/m³ Water as liquid 0 g/m³ 0 g/m³ 17 g/m³ 44.6 g/m³ 0 g/m³ 0 g/m³ Relative Humidity 50% Low 100% 100% 21% 36 % Per day Air volume 10,080 m³ 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ 1,260 m³ Water present 85,680 g 85,680 g 85,680 g 64,260 g 8,064 g 8,064 g Water as vapor 85,680 g 85,680 g 64,260 g 8,064 g 8,064 g 8,064 g Water as liquid 0 g 0 g 21,420 g = 21 liter 56,196 g = 56 liter 0 0 ## Conclusion
It's amazing how much water in generated in a pretty typical, maybe even 'small' compressed air system. Without air dryer, 21 liters of water is removed in the after cooler, and we end up with another 43 liters of water in our compressed air system! This water all condenses from water vapor into liquid water as the compressed air cools down in our piping system. If we add an air dryer to our system, it removes a whopping 56 liters of water per day. That's in addition to the 21 liters of water that was already removed in the after cooler of the air compressor. This example was with a pretty typical 45 kW air compressor and an ambient temperature of 20 °C with 50% relative humidity. At higher temperatures and humidity levels, the amount of water will be even higher. This simple example (with comprehensive calculations) shows how important it is to have a good drying system, together with a good condensate removal system. Take a moment to check your drying and condensate removing system today. Make sure that condensate traps are clean, condensate drains are working and all condensate tubes/piping is clean. Maybe you heard me talk about that time I visited a client and I found more than 1000 liters of water in his air receiver? In that particular case, all condensate tubes where connected to each other and the main line (it was just a small tube) was clogged. The air dryers was working, the condensate traps were working, there was just no way for the water to be removed.

Comments
No comments yet…
Log in or create an account to make a comment...