
Most of you guys reading this have experienced it at one point: water in your compressed air system. In fact, it’s one of the most common problems with compressed air systems. Back when I was still a compressor mechanic, I once made a visit to a client for routine maintenance. Part of the job is to drain the air receiver. Normally a half bucket full of water can be drained from a medium sized air receiver.  But a bucket wasn’t enough. After 3 buckets full of water, I got a bit worried.... It took some time to drain al the water, and it was almost 1000 liters (a lot of buckets!)! Not only was the air receiver full with water, most of the piping system was as well. Did someone mess up and connect the drinking water system to the compressed air system? Where did all the water come from? And, how do we get rid of the water and prevent problems like this? In this and the next article we will explore this mysterious miracle.
But a bucket wasn’t enough. After 3 buckets full of water, I got a bit worried.... It took some time to drain al the water, and it was almost 1000 liters (a lot of buckets!)! Not only was the air receiver full with water, most of the piping system was as well. Did someone mess up and connect the drinking water system to the compressed air system? Where did all the water come from? And, how do we get rid of the water and prevent problems like this? In this and the next article we will explore this mysterious miracle.
- Part 1 (this article): water in your compressed air - where does it come from?
- Part 2: Keeping your compressed air system water-free
Where’s the water coming from?
Let’s first answer the question “where is all that water in your compressed air system coming from”. The answer is surprisingly simple: the water comes from the ambient air. Air compressors suck it lots of compressed air and this ambient air contains water. It’s water vapor that’s present in the ambient air. All ambient air contains water vapor. The amount of water depends on where on this earth you live, the weather, temperature, etc. In a dry desert, there will be less water in the air. If you live in the tropics, there will be a lot of water in the air 
 Dry ambient air (me and a portable air compressor - Libya, long time ago) Wet ambient air (me and a portable air compressor - Malaysia, even longer time ago) The simple story is this: • Ambient air contains water • This air is sucked in by the air compressor and is compressed • As it’s compressed, the compressed air can’t hold the water anymore • Water vapor condenses into liquid water: water in your compressed air system Compressing wet air is like squeezing a wet sponge: water drips out . That’s the simple explanation. But why does the water condenses into liquid? Why can’t the compressed air hold as much water as the ambient air? How much water can we expect to be generated? To answer these questions, we need some background theory about water vapor and condensation, relative humidity and absolute humidity. ## Relative humidity and absolute humidity
Dry ambient air (me and a portable air compressor - Libya, long time ago) Wet ambient air (me and a portable air compressor - Malaysia, even longer time ago) The simple story is this: • Ambient air contains water • This air is sucked in by the air compressor and is compressed • As it’s compressed, the compressed air can’t hold the water anymore • Water vapor condenses into liquid water: water in your compressed air system Compressing wet air is like squeezing a wet sponge: water drips out . That’s the simple explanation. But why does the water condenses into liquid? Why can’t the compressed air hold as much water as the ambient air? How much water can we expect to be generated? To answer these questions, we need some background theory about water vapor and condensation, relative humidity and absolute humidity. ## Relative humidity and absolute humidity
The amount of water vapor in the air, or the humidity of the air can be expressed as either “relative humidity’ or ‘absolute humidity’. Absolute humidity is an absolute value, like grams of water per cubic meter. So for example, we could say “the absolute humidity of this air is 20 g/m3 (grams per cubic meter). So if we have 4 cubic meters of this air, it hold 4 * 20 = 80 grams of water vapor. Relative humidity is a percentage, like 70% relative humidity. You see, there’s a maximum of water vapor the air can hold. Relative humidity is a relative value, relative to the maximum holding capacity of the air. If the air holds the maximum amount of water, we say it's “100% relative humidity. We call that point the 'dew point' (we'll see why later!) If it holds 50% of the maximum it can hold, we call the 50% relative humidity.  Relative and absolute humidity example. (source: Compressed Air Confidence Course)
Relative and absolute humidity example. (source: Compressed Air Confidence Course)
For example: air can hold a maximum of 50 g/m3. At the moment, it holds 30 g/m3. In this case, the absolute humidity is 30 g/m3 and the relative humidity is 60% (30/50 * 100). ## Air temperature and dewpoint
The maximum amount of water vapor that air can hold is dependent on 1 thing only: the temperature of the air. Warm air can hold more water vapor than cold air. Much more. It’s the main mechanism behind clouds, rain … and water in your compressed air! So what happens if we heat up, or cool down air? The maximum amount of water vapor in the air (the holding capacity) will change! If we heat up the air, the holding capacity will increase. If we cool down the air, the holding capacity will decrease. What happens to the humidity of the air? The absolute humidity will stay constant. It will only change when we remove water vapor form the air or if we add water vapor to the air. Cooling and heating has no effect on the absolute humidity of the air. Relative humidity is another story. If we heat up the air, the holding capacity of that air increases. The means that since the absolute humidity stays the same, the relative humidity will drop. If we cool down the air, the holding capacity of the air decreases. As the air cools down, less and less water vapor can be kept in that air. 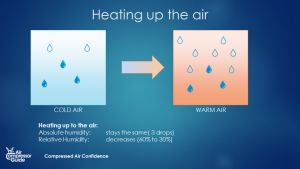 Heating up air increases the water vapor holding capacity - 5 drops to 10 drops in this example. (source: Compressed Air Confidence Course)
Heating up air increases the water vapor holding capacity - 5 drops to 10 drops in this example. (source: Compressed Air Confidence Course)
Heating up Cooling down Absolute humidity Stays constant Stays constant Relative humidity Goes down Goes If we keep cooling down the air, at one point, the amount of water that’s actually in the air is the same as the maximum holding capacity of that air. We call that temperature the **dewpoint**. So the dewpoint is a temperature. And it’s the temperature where the relative humidity is 100%. We call this ‘saturated air’: it can’t hold any more water than it already does. If the air cools down further, below the dewpoint, we have a problem: the air can’t hold the water vapor anymore and it will turn into liquid water: condensation! Dew point: the temperature at which the air is completely saturated with water vapor: the relative humidity (RH) is 100%. (source: [Compressed Air Confidence Course](https://courses.air-compressor-guide.com/course/compressed-air-confidence-course/))
Imagine you have a 5 liters in a 10 liter bucket, but the bucket becomes smaller and smaller. At some point, it will overflow. Same happens with the water vapor in the air as it cools down. ## Water vapor and your air compressor
This still doesn’t explain why there’s water in our compressed air. We are not cooling the air down, are we? If anything, the air heats up because of the compression. True. But here’s the thing: we are compressing the air. If we have a 7 bar compressed air system, we have compressed the ambient air 8 times (from 8 m3 to 1 m3 for example). (The pressure increased from 1 bar absolute to 8 bar absolute. Since we normally talk relative pressure in compressed air land, that’s from atmospheric pressure to 7 bar relative). The water vapor that was present in the 8m3 is now squeezed into 1 m3. By compressing the air 8 times, we are essentially increasing the relative humidity 8 times. The resulting compressed air is a theoretical 400% relative humidity (8* 50%). 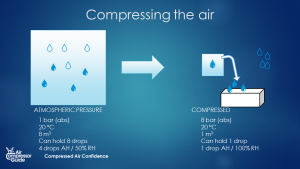 Compression of air - volume of air is reduced - the amount of water stays the same. The smaller volume can't hold the water anymore - water vapor condenses into liquid. (source: Compressed Air Confidence Course)
Compression of air - volume of air is reduced - the amount of water stays the same. The smaller volume can't hold the water anymore - water vapor condenses into liquid. (source: Compressed Air Confidence Course)
(this is with constant temperature – in reality compression increases the temperature. But if we give the air time to cool down, this story still holds true). What happens to the excess water? In condenses into liquid water. It’s like squeezing a sponge! The more water there was in the ambient air to begin with, the more water will condense in our compressed air system. And THAT is where all that water comes from! ## How do we remove water vapor from compressed air?
It's very important to remove water from the compressed air before it condenses into liquid water and flow towards your expensive machine or your end products, For this reason, every time compressed air is used for something that's sensitive to water, an air dryer is used to remove water vapor from the air. There are many different ways to remove water vapor from the air - but that's the subject of the next article on this subject. Coming soon!

Comments
No comments yet…
Log in or create an account to make a comment...